The particle model of matter refers to the position and motion of particles in different types of matter.
| State | Position and motion of particles |
|---|---|
| Solid | The particles are in fixed positions in a lattice structure. They are tightly packed together and can only vibrate within a particular position. |
| Liquid | Particles clump together but are not locked into fixed positions. The clumps are always large enough to fall to the bottom of any container due to gravity. They are close together. |
| Gas | Particles move quickly. They travel in straight lines until they hit another particle. They are widely spaced and do not interact except through collisions. |
| Plasma | Electrons have been stripped from the atoms to leave charged ions (nuclei) mixing with the free electrons. Plasma occurs around lightning and in the centre of the Sun. |
The temperature of an object is related to the motion or kinetic energy of all the particles it contains. If the particles are completely stationary they have no kinetic energy. The temperature of this object will be recorded as -273 degrees Celsius. This point is called ‘absolute zero’ or 0 degrees Kelvin.
Many people refer to the white mist above boiling water using the words ‘steam’ and ‘gas’ interchangeably. However, from a scientific perspective, it is important to distinguish clearly between the vapour state of water (a colourless gas) and the white mist which contains very small droplets of liquid water that form when the vapour cools (steam).
‘Change of state’ is usually given as one example of a physical (rather than a chemical) change and is reasonably clear as an example. Other examples, however, are not so clear. For example, the dissolving of salts in water is sometimes described as a physical change but also qualifies as a chemical change according to common definitions, since ionic bonds are broken. In this case, the dissolving of non-ionic compounds, such as sugar, in water is sometimes referred to as an example of a physical change, but this distinction is unlikely to help clarify the concept for students.
Chemical reactions involve the breaking down and creation of new pure substances. They involve chemical bonds being broken and reformed. Examples include rusting or the caramelisation of sugar into toffee. See Chemical reactions for more information.
Mixtures contain different substances mixed in varying proportions. They can be separated using a range of physical separation techniques.
- Filter paper acts as a fine sieve, letting through small particles and stopping the larger ones.
- Chromatography separates particles that have different solubilities.
- Magnets separate particles with magnetic properties.
- Centrifuging separate particles with different masses.
- Distillation separate particles with different boiling points.
Structure of an atom
Atoms consist of a small positively charged nucleus (containing positive protons and electrically neutral neutrons held together by a nuclear force) surrounded by a cloud of negatively charged electrons. Atoms are given their name based on the number of protons in the nucleus. Atoms with the same number of protons but different numbers of neutrons are called isotopes. Atoms with different numbers of electrons compared to protons are called ions.
Electrons are arranged in structured shell clouds that can be found in particular 3D locations around a nucleus. Electrons fill the innermost shell first (a maximum of 2 electrons). Successive electron shells and subshells are filled in order of energy levels. The further away from the nucleus, the higher the energy level.
Periodic table
Elements are listed in the periodic table in order of their atomic number i.e. the number of protons in their atoms: the Atomic Number. The listed Relative Atomic Masses are the average relative mass of the atoms found in the elements (on Earth), allowing for the different isotopes that are present. The rows are called ‘periods’ and the columns are called ‘groups’. Some groups have specific names; such as alkali metals, halogens, and noble gases.
The position of an element on the periodic table will depend on its properties and the number of electrons in its outer shell (valence electrons).
Elements in the same column have similar properties which generally grade down the group. The metals in the middle group, the transition elements, all tend to have similar properties, which are also shared by the actinides and lanthanides.
Elements with atomic numbers 1-98 have been found to exist in nature. Elements with higher atomic numbers have been produced artificially. Claims of new elements need to be confirmed by the International Union of Pure and Applied Chemistry before they can be officially named and included in the periodic table.
Note that a great majority of elements are metals with overall similar appearance and generally similar properties (although reactivity varies a lot). An interesting observation is that elements tend to become denser towards the bottom of the table because the atoms become more massive but do not change much in atomic volume. That is, elements at the bottom of the table are comprised of atoms that are significantly more massive, but which do not take up much more space.
Chemical reactions involve the breaking down and creation of new pure substances. They involve chemical bonds being broken and reformed. Examples include rusting or the caramelisation of sugar into toffee.
The rate a reaction occurs is related to both the kinetic energy of particles and the frequency of collisions. Reactions will occur more quickly if less energy is needed to break reactant bonds or the chemicals are already in a form that will react more easily, e.g. ionised in solution.
The main factors affecting reaction rate are:
- Concentration of reactants—the closer the particles are together, the more likely they will collide.
- Temperature of the reaction mixture—the higher the temperature, the faster the particles will move because they possess more kinetic energy.
- Size of particles of solid reactants—the smaller the particles, the larger the surface area exposed to the other reactant e.g. pieces of metal in an acid solution.
- Stirring—mixes reactants more quickly, increasing the frequency of collisions.
- Catalyst—increases the reaction rate by reducing the amount of energy required for the reaction to occur (lowering the activation energy). Catalysts often provide a surface on which reactions occur more readily. They are involved in the reaction, but are always released when the reaction is complete, so can be reused.
Combustion reactions
In the context of a bushfire, as a fire approaches, the radiant heat causes the wood and debris to reach a high temperature. This process of thermal degradation leads to dehydration of the material and the breakdown of the cellulose and lignin. This released further heat and pyrolysis (the production of volatile gases and tar). If this continues, the plant matter can ignite, starting the process of combustion.
One of the main forms of fuel in Australia is the highly flammable eucalyptus trees whose oil is released during the process of thermal degradation stage. When these leaves and bark undergo pyrolysis, the result can be rapid and explosive.
A combustion reaction is one where a fuel reacts with oxygen gas releasing energy in the form of heat and light. The most common general equation for a combustion reaction is:
$$\require{mhchem}\ce{ Fuel (hydrocarbon) + Oxygen -> Carbon\,dioxide + Water}$$
In these examples, the mass of the fuel decreases as the water and carbon dioxide are released as a gas. This can be shown through a fire triangle.
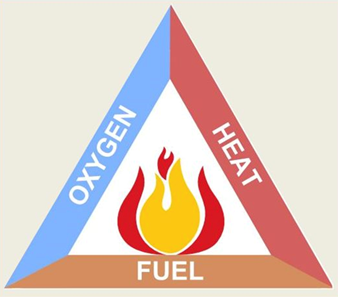
This is not the only form of combustion. When hydrogen burns in the presence of oxygen, only water is produced (no carbon dioxide).
$$\ce{2H2 + O2 -> 2H2O}$$
When a metal burns in the presence of a metal, a metal oxide is produced.
$$\ce{Metal + Oxygen -> Metal\,oxide}$$
Further information will be provided in the future.
| Alternative conception | Accepted conception |
|---|---|
| Energy is released in combustion because of the breaking of bonds. | Energy needs to be absorbed to break the bonds between the fuel and oxygen. Energy is then released when new bonds are formed. In combustion, the amount of energy released when forming bonds is greater than the amount of energy needed to break the bonds in the fuel and oxygen. Therefore, heat is needed to start the reaction, but once started, heat and light are released. |
The mass will decrease in a combustion reaction because:
| Combustion causes a decrease in mass because carbon dioxide and water are produced in a gas form. |
| Air is weightless or has negative weight. | Air has mass and can be affected by gravity. Therefore, air has weight. |
| There is air between air particles/molecules. | There is space, not air, between particles. |
| If a solid is heated, the particles get bigger. | At higher temperatures, molecules gain more kinetic energy, move more, and take up more space. |
| The distance between molecules in a liquid is halfway between those in a solid and gas. | Molecules in a liquid are close together but can move freely around each other. |
| Gas has lots of moving molecules. | Most of a gas has empty space between the molecules (unless it is under high pressure in a confined space). |
| Molecules have the same properties as the solid, liquid, or gas they make up. | Solid, liquid, or gaseous materials have unique properties that are dependent on the intermolecular bonds between the molecules. This is different from the properties of the individual molecules. |
| Boiling and evaporating are the same thing. | Boiling happens at a set temperature for a pure substance. Evaporation can happen at any temperature if a particle can gain enough kinetic energy to escape the surface. |
| Boiling is the maximum temperature a substance can reach. | The boiling point is the maximum temperature that a substance can be classified as a liquid. |
| Objects float in water because they are lighter. | Objects float because they are less dense ($density = \frac{mass}{volume}$) than water. |
| Liquids of high viscosity are also high density. | Viscosity is a measure of the particles in a liquid’s ability to move around each other. |
| Liquids rise in a straw because of ‘suction’. | The suction causes a decrease in pressure, which forces liquid into and up the straw. |
| Ice cannot change temperature. | Pure ice forms at 0 degrees Celsius. It can become much colder than that once formed. |
| The bubbles in boiling water contain ‘air’. | Bubbles are formed in heated water when the water liquid forms water vapor. |
| Heating a substance always means increasing its temperature. | The transfer of heat/thermal energy will typically increase the temperature of a substance except when the substance is changing its state. At that point, the energy does work to break the intermolecular bonds and temporarily stabilises the temperature. |
| A heat insulator warms things up. | An insulator prevents the transfer of heat/thermal energy. |
| Metals attract cold faster than wooden objects. | Metals conduct thermal energy faster than wood. Metal does not attract thermal energy. |
| The space between atoms contains air. | Atoms can share electrons, but there are no ‘air molecules’ between atoms. |
| Iron atoms have the same properties as iron metal. | Iron atoms cannot melt like iron metal. Bulk elements have emergent properties due to their bonding arrangements. |
| Silver atoms are silver in colour. | Atoms do not have a colour. |
| Chemical bonds store energy. | Chemical bonds contain potential energy. Energy is required to break the bonds. The difference in energy between the reactant molecules and the product molecules can result in the release or absorption of energy from the surrounding environment. |
| Atoms have electrons circling them like planets around a star. | Electrons are arranged in structure shell clouds that can be found in particular 3D locations around a nucleus. |
| Electron pairs are shared equally in a covalent bond. | Some nuclei have a higher electronegativity than others and therefore electrons are more likely to spend time closer to that nucleus. |
| Molecules of solids are hard, and molecules of gases are soft. | The properties of a material (multiple atoms chemically bonded) are emergent and different from individual atoms. |
| Molecules of solids are larger than molecules of gases. | Atom size is determined by the number and arrangement of sub-atomic particles (protons, neutrons, and electrons). |
References
AITSL. (n.d.). Resource. AITSL. https://www.aitsl.edu.au/tools-resources/resource/dispelling-scientific-misconceptions-illustration-of-practice
Allen, M. (2019). Misconceptions in Primary Science 3e. McGraw-hill education (UK).
Ideas for Teaching Science: Years 5-10. (2014, April 14). Resources for Teaching Science. https://blogs.deakin.edu.au/sci-enviro-ed/years-5-10/
Pine, K., Messer, D., & St. John, K. (2001). Children's misconceptions in primary science: A survey of teachers' views. Research in Science & Technological Education, 19(1), 79-96.
Redhead, K. (2018). Common Misconceptions. Primary Science Teaching Trust. https://pstt.org.uk/resources/common-misconceptions/
University of California. (2022, April 21). Correcting misconceptions - Understanding Science. Understanding Science - How Science REALLY Works... https://undsci.berkeley.edu/for-educators/prepare-and-plan/correcting-misconceptions/

